Synthesis and doping of high-temperature resistant spinel nano pigments: A review
- 1 Department of Materials Engineering, Naghshejahan Institute of Higher Education, Baharestan, Isfahan, Iran
- 2 Advanced Materials Research Center, Department of Materials Engineering, Najafabad Branch, Islamic Azad University, Najafabad, Iran
- 3 Department of Inorganic Chemistry, Vilnius University, Naugarduko 24, Vilnius LT-03225, Lithuania
Abstract
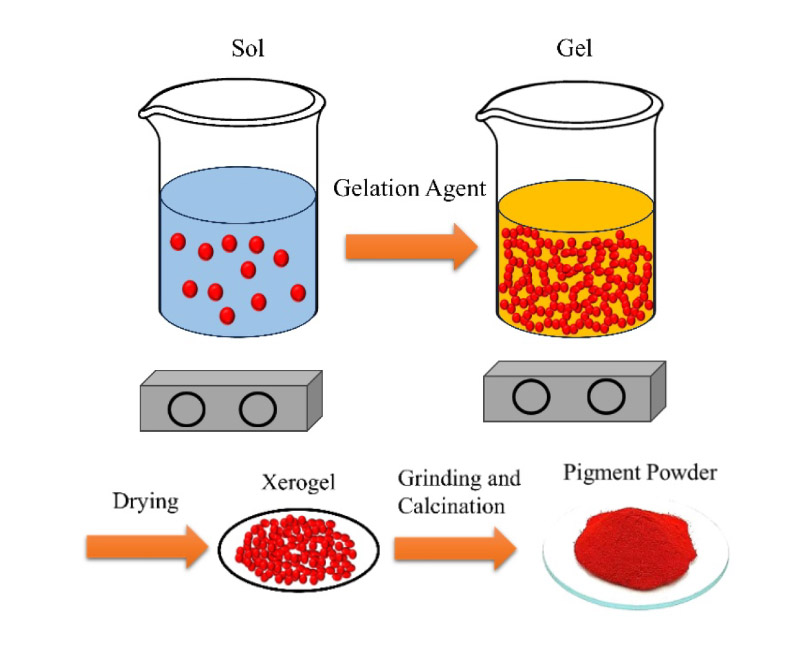
Spinel nano-pigments are high-performance super small particles, combining the stable properties of the spinel structures with the high activity of Nanomaterials. While entrapment of highly toxic yet beautiful chromophores in the spinel structure diminishes the toxicity and improves the thermal resistance, a high surface area provided by the nano-scale pigments results in a uniform bright coating with a sufficiently high color strength and enhanced light transmission. Although the spinel nano pigments are promising for the coating and ceramic industries, the applications are limited, mostly due to the required high sintering temperature. Various synthesis processes have been tried for these pigments with the sol-gel method being the most frequent one. Many elements have been considered as dopants for these spinel systems to enhance, change, or improve the optical and physical properties. This comprehensive review aims to summarize the work done in this field, covering almost 20 years of research dedicated to the synthesis and doping of spinel nano pigments.
Downloads
References
Copyright (c) 2024 Rayehe Tavakolipour, Reza Pournajaf, Egle Grazenaite

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.