Crystallization behavior and ionic conductivity of NASICON type glass-ceramics containing different amounts of B2O3
- 1 School of Metallurgy & Materials Engineering, Iran University of Science and Technology, Tehran, Iran
- 2 Ceramic Department, Materials and Energy Research Center (MERC), Alborz, Iran
Abstract
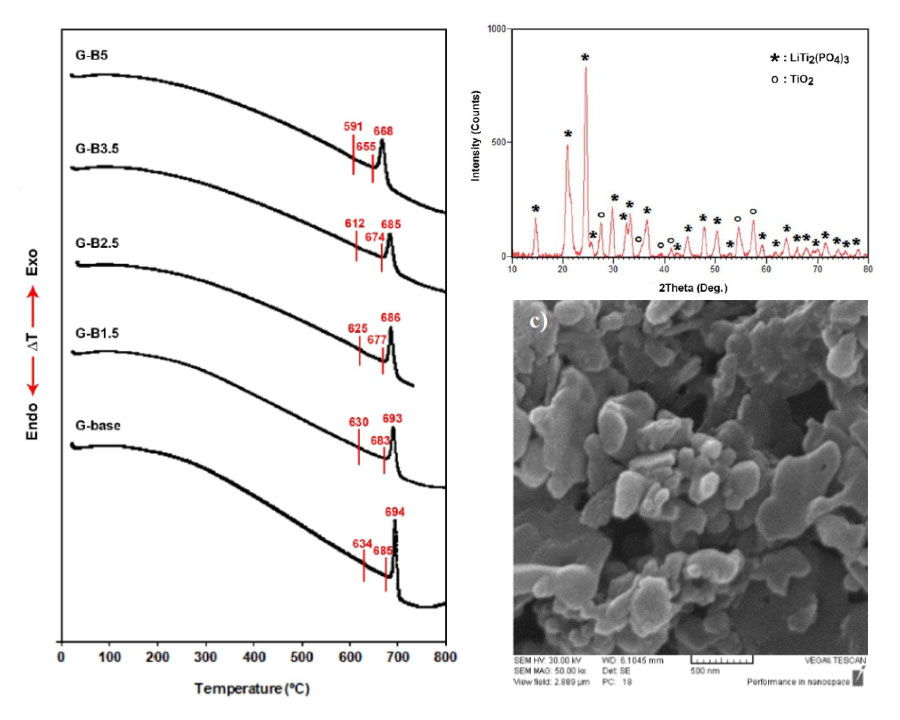
In this research, glass-ceramics belonging to the system of Li2O-TiO2-P2O5 were prepared by the addition of different amounts of B2O3. The glass formation ability of the starting glass materials along with the crystallization trend as well as ionic conductivity of the corresponding glass-ceramics were also examined. Starting glasses were obtained through the melt quenching technique and glass-ceramics specimens were prepared through one-step heat treatment. The glass-ceramic samples were then examined through X-ray diffractometry, differential thermal analysis, electrochemical impedance spectroscopy, and scanning electron microscopy. According to the obtained results, the addition of a 2.5 mol% of B2O3 to the glass composition led to a sharp increase in ionic conductivity at room temperature. So that the bulk conductivity of the specimen heat treated at 950 °C for 2 h was measured to be 1.17 × 10-3 Scm-1, which was 10 times bigger than that of the base glass-ceramic with no additive. It also decreased the crystallization temperature and viscosity of the parent glass, resulting in increased crystallinity while further addition of B2O3 drained the conductivity and crystallinity of glass-ceramics.
Downloads
References
Copyright (c) 2023 Banafsheh Zarabian, Bijan Eftekhari Yekta, Sara Banijamali

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.