Optimum temperature, time and atmosphere of precursor pyrolysis for synthesis of B4C ceramics
- 1 Ceramics Department, Materials and Energy Research Center (MERC), Karaj, Iran
- 2 Materials and energy research center
Abstract
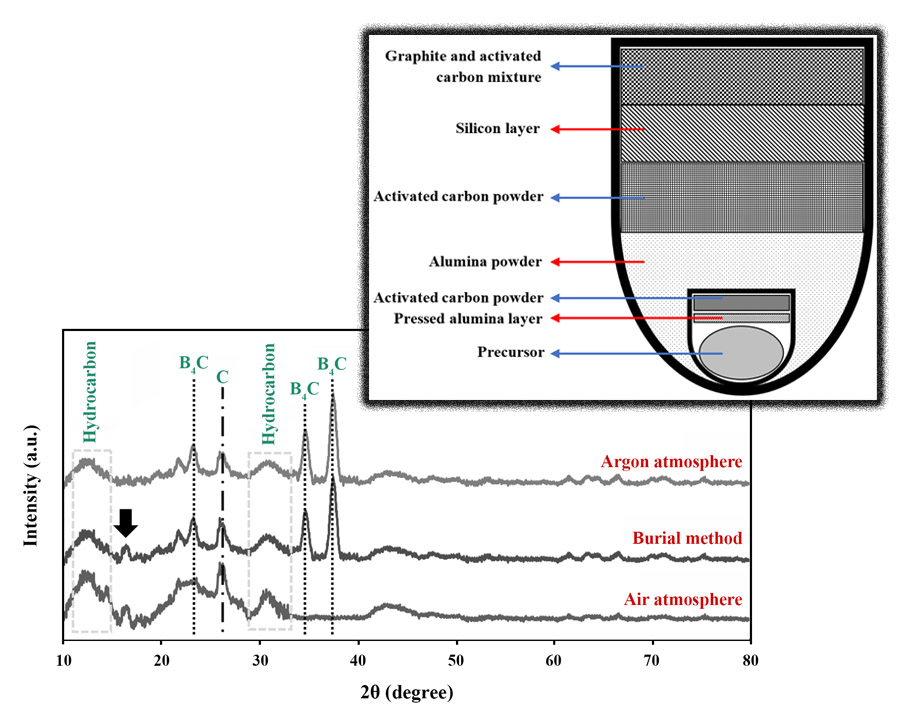
In this paper, the variables of the pyrolysis operation such as temperature, time, and atmosphere were studied and optimized. At first, the effect of increasing pyrolysis time at lower temperatures was investigated to understand the mutual influence of pyrolysis time and temperature in enhancing the efficiency of B4C synthesis. Then, three pyrolysis atmospheres were selected to find the optimal conditions: burial method in a box furnace (air), pyrolysis in a tubular furnace (argon), and pyrolysis in a box furnace (air). The pyrolyzed powders were finally located inside the tubular furnace at 1500 °C for 4 h under an argon atmosphere to synthesize B4C ceramics. X-ray diffractometry (XRD) was employed to determine the optimal processing conditions. The temperature of 600 °C and the holding time of 2 h were selected as the optimal pyrolysis conditions. Meanwhile, the burial method was chosen as the best atmosphere despite having a higher percentage of impurity because of the much lower cost compared to the argon atmosphere.
Downloads
References
Copyright (c) 2022 Seyed Faridaddin Feiz, Leila Nikzad, Hudsa Majidian, Esmaeil Salahi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.