Effects of glucose pretreatment and boric acid content on the synthesizability of B4C ceramics
- 1 Ceramics Department, Materials and Energy Research Center (MERC), Karaj, Iran
Abstract
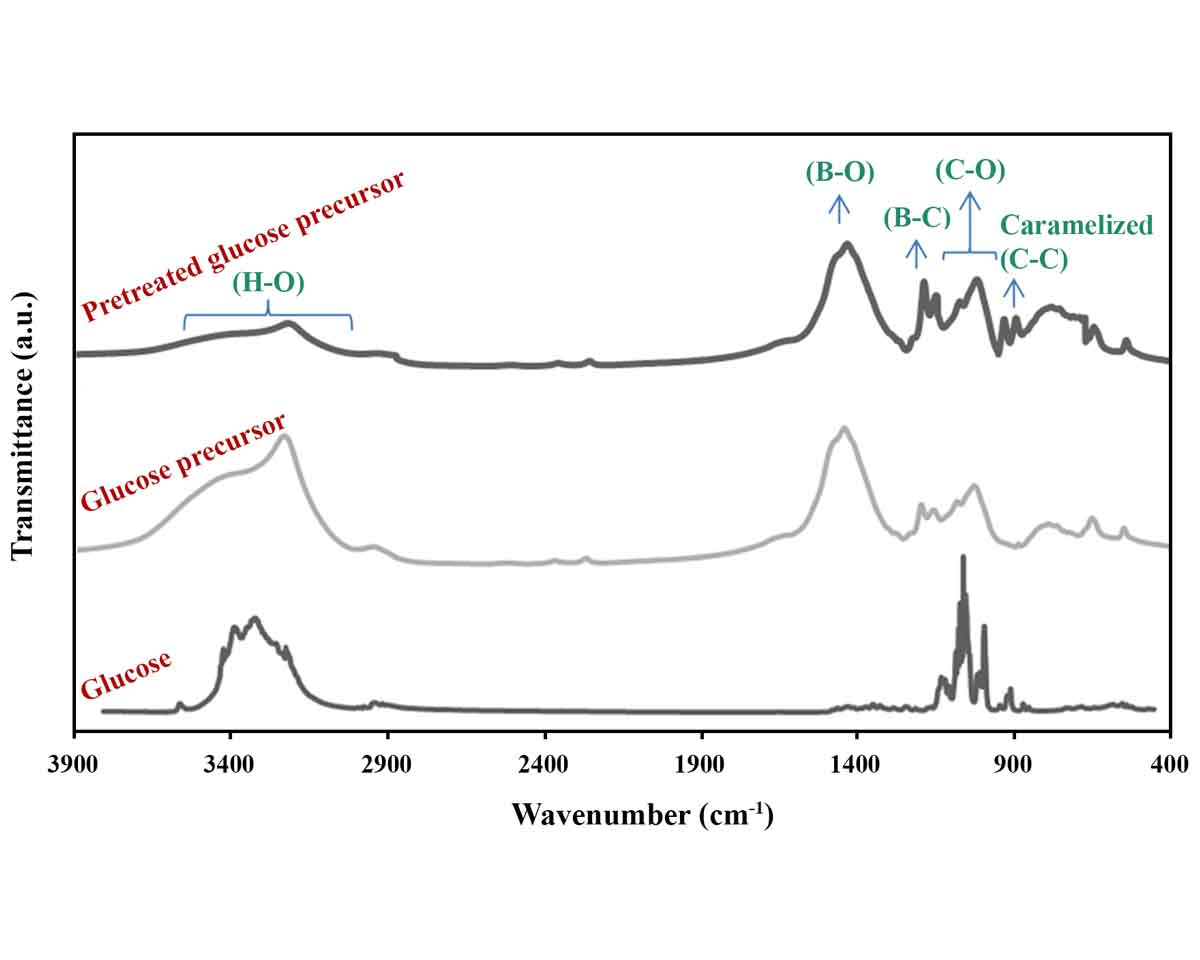
Synthesis of boron carbide (B4C) as one of the hardest materials on planet Earth is of particular importance due to its wide range of industrial and engineering applications. For this purpose, boric acid and polymers can be used as the boron and carbon sources, respectively. From the family of saccharides in polymeric materials, glucose has shown the best performance for the synthesis of B4C. In this research, untreated and pretreated (caramelized by heating) glucose precursors were selected and mixed with boric acid for subsequent pyrolysis and synthesis processes. X-ray diffractometry and Fourier transform infrared spectroscopy confirmed that heat-treated glucose is a better carbon precursor for B4C synthesis. In order to evaluate the effect of the amount of boric acid, more than its stoichiometric ratio, additional amounts of boric acid (10–40%) were also examined and the excess amount of 30% was determined as the optimal value.
Downloads
References
Copyright (c) 2022 Seyed Faridaddin Feiz, Leila Nikzad, Hudsa Majidian, Esmaeil Salahi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright
Authors are the copyright holders of their published papers in Synthesis and Sintering, which are simultaneously licensed under a Creative Commons Attribution 4.0 International License. The full details of the license are available at https://creativecommons.org/licenses/by/4.0/.
All papers published open access will be immediately and permanently free for everyone to read, download, copy, distribute, print, search, link to the full-text of papers, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose without any registration obstacles or subscription fees.